Featured
-

Meeting (and subverting) college expectations
Editorials featured in the Forum section are solely the opinions of their individual authors. When I was accepted into Carnegie Mellon University, I never got the opportunity to fly out…
-

Thousands gather outdoors to witness rare solar eclipse
By Sam Bates and Arden Ryan Just after 2 p.m. on April 8, the Pittsburgh…
-

A voter’s guide to the upcoming PA primary election
On April 23, Pennsylvania will hold its primary elections. The winners of these races will…
-
Thousands gather outdoors to witness rare solar eclipse
By Sam Bates and Arden Ryan Just after 2 p.m. on April 8, the Pittsburgh sky began to darken, creatures…
-
Students enjoy Carnival despite rainy weather
By Nina McCambridge Last week, tents and rides began going up on the Cut as students lugged wood to Midway.…
-
What I did this weekend
Before you read, look at this and this Calvin and Hobbes strip, they’re loosely the inspiration. I give you the…
-
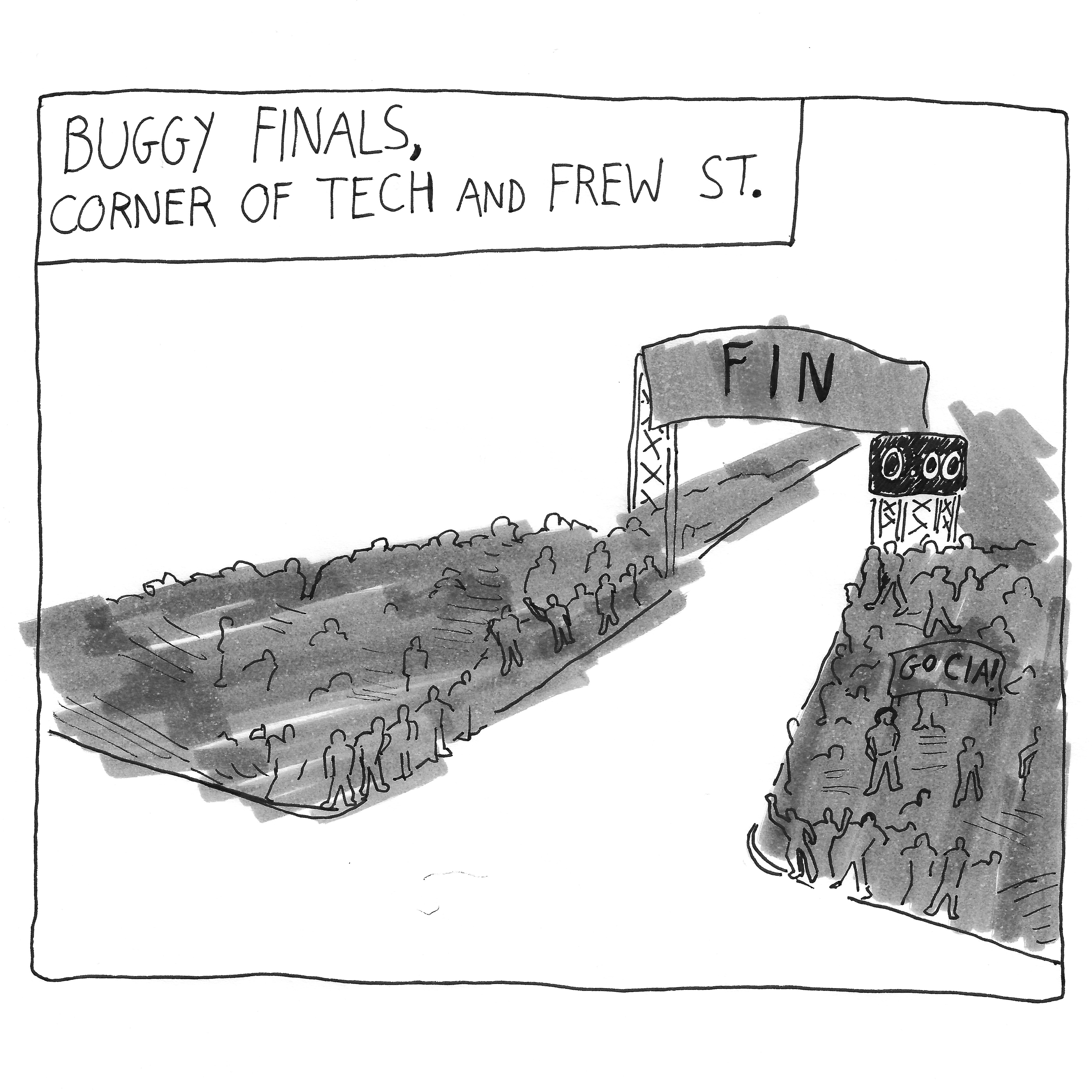
Buggy Sweepstakes final race results
After a rainy Friday canceled the first day of Buggy rolls, Raceday 2024 started and finished on Saturday, April 13. …
-
‘The Little Mermaid’ review
By Savannah Milam This Carnival weekend, Scotch’n’Soda’s production of Disney’s “The Little Mermaid” took audiences on a journey under the…
-

Softball beats NYU twice in doubleheader
Women’s golf and softball dominated, while other CMU Sports also excelled!
-

Bill Nye the Science Guy visits CMU for Carnival
On Thursday, April 11 at 6:30 p.m., two long lines wound through the Cohon University…
-

The science behind predicting solar and lunar eclipses
When the solar eclipse happened in Pittsburgh last Monday, we knew everything about it: The…
-

Video game-themed booths line midway, SDC wins best overall
Every year in the week leading up to Carnival, many organizations on campus call all…
-

Thousands gather outdoors to witness rare solar eclipse
By Sam Bates and Arden Ryan Just after 2 p.m. on April 8, the Pittsburgh…
-

A voter’s guide to the upcoming PA primary election
On April 23, Pennsylvania will hold its primary elections. The winners of these races will…
-

CMU holds Spring farmers market
On Tuesday, April 9, the Spring Farmers Market returned to Carnegie Mellon. Vendors at the…
-

Meeting (and subverting) college expectations
Editorials featured in the Forum section are solely the opinions of their individual authors. When…
-

EdBoard: Welcome to the Carnival, folks
By The Tartan Editorial Board Editorials featured in the Forum section are solely the opinions…
-
There’s too much merch!
Editorials featured in the Forum section are solely the opinions of their individual authors. No…
-

Novel-tea: How many chili peppers?
Editorials featured in the Forum section are solely the opinions of their individual authors. I…
-

Robotic Institute researchers showcase projects
On Friday, April 12, the Robotics Institute offered tours of some of its laboratories. First,…
-

Bill Nye the Science Guy visits CMU for Carnival
On Thursday, April 11 at 6:30 p.m., two long lines wound through the Cohon University…
-

The science behind predicting solar and lunar eclipses
When the solar eclipse happened in Pittsburgh last Monday, we knew everything about it: The…
-

The lifespan of game consoles: From birth to obsolescence
On April 8, Nintendo shut down online services for the editions of the 3DS and…
-

Case Watch
BOOOOO SPARTANS
-

NBA Regular season wrap up: surprises
As the NBA regular season wraps up, let’s reflect on some favorite surprises.
-

Foul Play: The NCAA
The University of Connecticut’s sixth men’s basketball national title highlights a tremendous year in NCAA…
-

Buggy Sweepstakes final race results
After a rainy Friday canceled the first day of Buggy rolls, Raceday 2024 started and…
-

What I did this weekend
Before you read, look at this and this Calvin and Hobbes strip, they’re loosely the…
-

Capitalism (advertising) isn’t fun anymore
Commercials aren’t fun anymore. I promise I’m not going to rant about how they put…
-
The tired, the poor, the huddled masses yearning to be free
“One star is for Alaska… One star is for Nebraska… One star is North Dakota……
-

Standing on the corner where the sidewalk ends
Have you ever read Shel Silverstein? If you haven’t, I’d recommend sampling a few of…